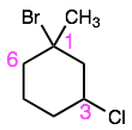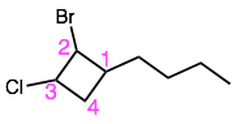Structure
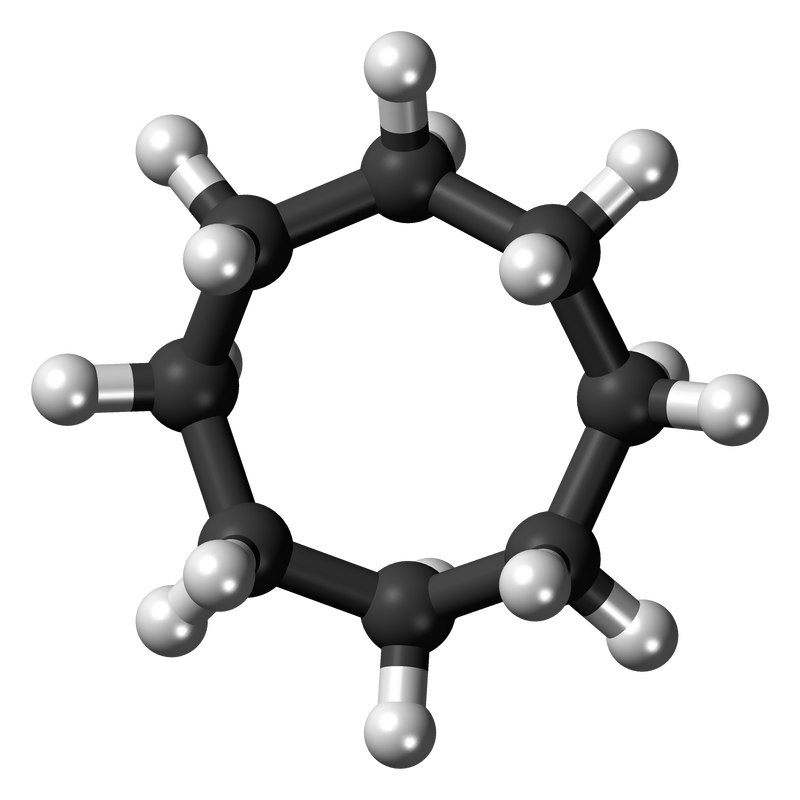
Alkanes are compounds that consist of only single bonded carbons and hydrogens. Cycloalkanes are alkanes that consist of at least three carbon atoms linked together to form a structural ring (hence the prefix 'cyclo-') as shown below.
Naming Rules
The rules for naming rings is similar but slightly different from linear alkanes and haloalkanes. Simple cycloalkanes,which are those without any additional branches have the generic formula of CnH(2n) and are named by adding the prefex cyclo- to the name of the corresponding alkane chain with the same number of carbons as the ring.
The following steps must be followed in order.
Step 1
Determine the parent chain
The ring will be the parent chain (even if there is a longer linear chain attached to the ring). The ring is named by the number of carbons (as you would with alkanes) but with the prefix cyclo
e.g. cyclohexane (six-carbon ring) or cyclooctane (eight-carbon ring)
Step 2
Number the parent ring
Number the ring starting from the carbon with the branch (referred to as substituent in video) lowest in the alphabet. Number in the direction that give the lower overall substituent numbers.
Step 3
Name any groups attached to the parent ring
-carbon groups are named methyl, ethyl, propyl etc.
-halogen groups are named fluoro, chloro, bromo etc.
Step 4
Group together repeated branches/groups using the prefixes di-, tri-, tetra- etc
Step 5
List the groups in alphabetical order
Step 6
Write out the name in the following format:
number, number-prefixgroup-number-prefixgroup parentchain
Examples
Name these cycloalkanes
The following steps must be followed in order.
Step 1
Determine the parent chain
The ring will be the parent chain (even if there is a longer linear chain attached to the ring). The ring is named by the number of carbons (as you would with alkanes) but with the prefix cyclo
e.g. cyclohexane (six-carbon ring) or cyclooctane (eight-carbon ring)
Step 2
Number the parent ring
Number the ring starting from the carbon with the branch (referred to as substituent in video) lowest in the alphabet. Number in the direction that give the lower overall substituent numbers.
Step 3
Name any groups attached to the parent ring
-carbon groups are named methyl, ethyl, propyl etc.
-halogen groups are named fluoro, chloro, bromo etc.
Step 4
Group together repeated branches/groups using the prefixes di-, tri-, tetra- etc
Step 5
List the groups in alphabetical order
Step 6
Write out the name in the following format:
number, number-prefixgroup-number-prefixgroup parentchain
Examples
Name these cycloalkanes
Watch the video below for a more complex example
Physical Properties
Similar to alkanes, cycloalkanes are covalent, non-polar molecules, therefore when bonding they use Van der Waals forces. Due to this type of bonding, the smaller cycloalkanes such as cyclopropane and cyclobutane are gases and the rest are liquids and solids.
As the number of carbon atoms in the molecule increase, the Van der Waals forces are stronger and therefore will have higher melting and boiling points. In fact, cycloalkanes have higher boiling points, melting points and densities than linear alkanes.
Also, cycloalkane VDW forces are stronger than alkanes as the ring shape allows for greater area of contact and they are more reactive.
Cycloalkanes are less dense than water and have low solubility, and lower cycloalkanes are especially flammable in oxygen.
Also, cycloalkanes are considered saturated molecules due to single alkane bonds.
As the number of carbon atoms in the molecule increase, the Van der Waals forces are stronger and therefore will have higher melting and boiling points. In fact, cycloalkanes have higher boiling points, melting points and densities than linear alkanes.
Also, cycloalkane VDW forces are stronger than alkanes as the ring shape allows for greater area of contact and they are more reactive.
Cycloalkanes are less dense than water and have low solubility, and lower cycloalkanes are especially flammable in oxygen.
Also, cycloalkanes are considered saturated molecules due to single alkane bonds.
Chemical Properties
The chemical properties of cycloalkanes are similar to those of the corresponding open chain compounds in alkanes
- Low reactivity due to strong carbon-carbon and carbon-hydrogen single bonds with the exception of smaller rings such as Cyclopropane
- The smaller the ring, the more likely it is to undergo reactions due to the carbon-carbon bond angle. For example Cyclopropane is the most reactive as it has the smallest bond angle of 60 degrees.
- Inert substances meaning that they do not undergo many reactions. Common reactions that they do undergo include:
1) Combustion
Or also known as burning in oxygen. Example of an oxidation reaction- hydrocarbons are oxidised. Cycloalkanes react with oxygen to form carbon dioxide and water.
2) Halogenation
Molecular halogens (Cl, F, Br) react with cycloalkanes in the presence of ultraviolet light or heat to form alkyl halides. Halogenation is a substituion reaction, in which a hydrogen in the cycloalkanes is replaced by a halogen.
.
- Low reactivity due to strong carbon-carbon and carbon-hydrogen single bonds with the exception of smaller rings such as Cyclopropane
- The smaller the ring, the more likely it is to undergo reactions due to the carbon-carbon bond angle. For example Cyclopropane is the most reactive as it has the smallest bond angle of 60 degrees.
- Inert substances meaning that they do not undergo many reactions. Common reactions that they do undergo include:
1) Combustion
Or also known as burning in oxygen. Example of an oxidation reaction- hydrocarbons are oxidised. Cycloalkanes react with oxygen to form carbon dioxide and water.
2) Halogenation
Molecular halogens (Cl, F, Br) react with cycloalkanes in the presence of ultraviolet light or heat to form alkyl halides. Halogenation is a substituion reaction, in which a hydrogen in the cycloalkanes is replaced by a halogen.
.